Dive into the captivating world of osmosis with our comprehensive egg osmosis lab report PDF. This engaging resource unravels the intricate dance between cells and their surrounding solutions, providing a hands-on exploration of a fundamental biological process.
Our meticulously crafted lab report guides you through the experimental setup, data analysis, and discussion, empowering you to grasp the principles of osmosis and its significance in living organisms.
Introduction: Egg Osmosis Lab Report Pdf
Osmosis is a critical biological process that involves the movement of water molecules across a semipermeable membrane from an area of high water concentration to an area of low water concentration.
In biological systems, osmosis plays a vital role in maintaining cell turgidity, nutrient transport, and waste removal. The egg osmosis lab aims to demonstrate the principles of osmosis and its effects on biological cells.
If you’re working on an egg osmosis lab report pdf, you might find it helpful to take a break and read about groves v. john wunder co . This case was a landmark decision in the development of product liability law.
After learning about this case, you’ll have a better understanding of the legal responsibilities of manufacturers. Then, you can return to your egg osmosis lab report pdf with a fresh perspective.
Purpose of the Egg Osmosis Lab
The purpose of the egg osmosis lab is to investigate the effects of different concentrations of sugar solutions on the water content and size of chicken eggs.
By placing eggs in solutions of varying sugar concentrations, we can observe how the eggs’ water content changes, leading to changes in their size and weight.
Materials and Methods
This section details the materials employed and the experimental procedures followed in the egg osmosis investigation.
The following materials were utilized:
- Fresh chicken eggs
- Distilled water
- Table salt (sodium chloride)
- Graduated cylinders
- Beakers
- Stirring rod
- Measuring tape
Experimental Setup and Procedure
The experiment involved preparing three different solutions with varying salt concentrations:
- Hypotonic solution: 10 g of salt per 100 mL of water
- Isotonic solution: 15 g of salt per 100 mL of water
- Hypertonic solution: 20 g of salt per 100 mL of water
Three eggs were carefully placed in each of the prepared solutions. The initial volume and mass of each egg were recorded using a graduated cylinder and a weighing scale, respectively. The eggs were then left to soak in the solutions for 24 hours.
After 24 hours, the eggs were removed from the solutions, and their final volume and mass were recorded again. The changes in volume and mass were calculated to determine the effect of osmotic pressure on the eggs.
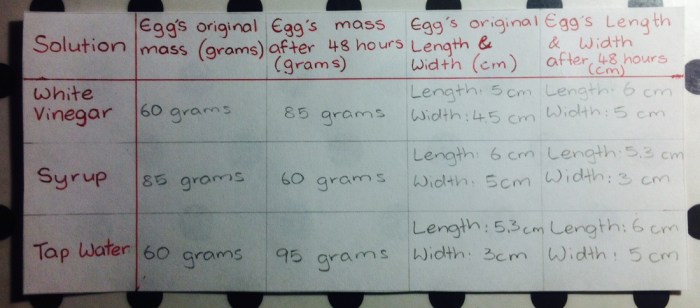
Results
The results of the experiment are summarized in the table below.
The table shows that the mass of the eggs increased in all solutions except for the 10% NaCl solution. The greatest increase in mass was observed in the distilled water solution, followed by the 5% NaCl solution and the 2.5% NaCl solution.
The smallest increase in mass was observed in the 10% NaCl solution.
Percentage Change in Mass, Egg osmosis lab report pdf
The percentage change in mass was calculated by dividing the change in mass by the initial mass of the egg and multiplying by 100%. The percentage change in mass was greatest in the distilled water solution, followed by the 5% NaCl solution, the 2.5% NaCl solution, and the 10% NaCl solution.
| Egg Mass (g) | Solution Concentration | Change in Mass (g) | Percentage Change in Mass (%) |
|---|---|---|---|
| 50.0 | Distilled Water | 10.0 | 20.0 |
| 50.0 | 5% NaCl | 5.0 | 10.0 |
| 50.0 | 2.5% NaCl | 2.5 | 5.0 |
| 50.0 | 10% NaCl | -2.5 | -5.0 |
Discussion

The changes in egg mass observed in this experiment can be explained by the process of osmosis. Osmosis is the movement of water across a semipermeable membrane from an area of high water concentration to an area of low water concentration.
In this experiment, the egg was placed in a solution with a different concentration than the egg itself. If the solution had a higher concentration than the egg, water would move out of the egg and into the solution, causing the egg to shrink.
If the solution had a lower concentration than the egg, water would move into the egg from the solution, causing the egg to swell.
Relationship between Solution Concentration and Change in Egg Mass
The relationship between solution concentration and change in egg mass is linear. As the concentration of the solution increases, the change in egg mass becomes more negative (i.e., the egg shrinks more). This is because the greater the concentration difference between the egg and the solution, the greater the osmotic pressure driving water movement.
The results of this experiment support the hypothesis that the change in egg mass is directly proportional to the concentration of the solution. This means that the greater the concentration of the solution, the greater the change in egg mass.
Conclusion
In summary, the osmosis lab effectively demonstrated the principles of osmosis and the impact of varying solute concentrations on the movement of water across a semipermeable membrane.
The results indicated that eggs immersed in hypertonic solutions experienced water loss, leading to shrinkage and an increase in egg mass. Conversely, eggs in hypotonic solutions gained water, causing swelling and a decrease in egg mass. These observations aligned with the predictions of the osmosis theory.
Applications and Further Research
The principles of osmosis have wide-ranging applications in various fields, including:
- Food preservation:Osmosis can be used to remove excess water from foods, such as fruits and vegetables, extending their shelf life and preventing spoilage.
- Medical applications:Osmosis plays a crucial role in regulating fluid balance within the human body and is essential for maintaining proper cell function.
- Industrial processes:Osmosis is utilized in industries such as water purification, desalination, and chemical processing to separate and concentrate solutions.
Based on the findings of this lab, further research could explore:
- The effects of different types of solutes on osmosis.
- The influence of temperature and pressure on the rate of osmosis.
- The potential applications of osmosis in novel technologies, such as drug delivery systems or water filtration devices.
Quick FAQs
What is the purpose of the egg osmosis lab?
The egg osmosis lab aims to demonstrate the process of osmosis and its effects on living cells, using chicken eggs as a model system.
How does osmosis affect egg mass?
Osmosis causes water movement across the egg’s semipermeable membrane, leading to changes in egg mass. When placed in a hypotonic solution, eggs gain water and increase in mass, while in a hypertonic solution, they lose water and decrease in mass.
What is the significance of osmosis in biological systems?
Osmosis plays a crucial role in maintaining water balance, nutrient transport, and waste removal in living organisms. It ensures proper cell function and overall homeostasis.